Background: Approximately 40% of patients (pts) with non-Hodgkin lymphoma (NHL) have disease that will relapse or is refractory to chemotherapy and/or immunotherapies; management of these pts remains a challenge. Although follicular lymphoma (FL) generally responds well to first-line treatment (tx), this disease is characterized by frequent relapses with shorter intervals between tx lines. Pts with early relapse (ER) (progressive disease <2 y after initial diagnosis) and those who are double-refractory (DR) to both rituximab and chemotherapy, have particularly poor outcomes. Avadomide (CC-122) is a small molecule oral agent that induces cereblon-mediated degradation of the transcription factors Ikaros and Aiolos and promotes antilymphoma activity. Results from the dose-escalation part of the CC-122-NHL-001 study demonstrated preliminary antitumor activity of avadomide in combination with obinutuzumab in pts with relapsed and/or refractory (R/R) FL. Here, we report long-term safety and efficacy results from the dose-escalation and dose-expansion parts of the CC-122-NHL-001 study in pts with R/R FL.
Methods: CC-122-NHL-001 (NCT02417285) is an ongoing, open-label, phase 1b study of avadomide in combination with obinutuzumab conducted at 8 sites in 3 European countries with dose-escalation and dose-expansion parts in R/R FL. Eligible pts (age ≥18 y) had histologically confirmed, CD20-positive R/R NHL. Pts with FL (grade 1, 2, or 3a) had ≥1 prior standard regimen. Avadomide active ingredient in capsule (1.0-4.0 mg) or formulated capsules (3.0 or 4.0 mg) was administered orally once daily on days 1-5 followed by 2 days off (5/7-day schedule) every week of each 28-day cycle. Obinutuzumab 1000 mg was given intravenously on days 2, 8, and 15 of cycle 1 and on day 1 of cycles 2-8. Primary objectives were to determine the safety and tolerability of the combination, including the non-tolerated dose, maximum tolerated dose, and recommended phase 2 dose (RP2D). Response was assessed using Cheson 2007 criteria every 2 cycles to cycle 6, then every 3 cycles to cycle 12, and every 6 cycles thereafter. Median duration of response (mDOR) and median progression-free survival (mPFS) were assessed by Kaplan-Meier estimates.
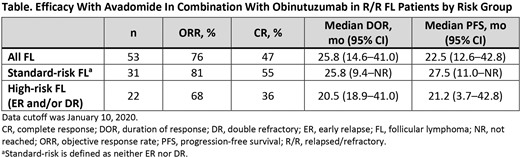
Results: As of January 10, 2020, 73 pts with R/R NHL were enrolled and treated, including 19 with R/R diffuse large B-cell lymphoma, 1 with marginal zone lymphoma, and 53 with R/R FL; all 35 pts in the dose-expansion part of the study had R/R FL. The median age among treated pts was 61 y (range, 26-83), the median number of prior antilymphoma therapies was 3 (range 1-11), and 26 pts (36%) had prior autologous stem cell transplantation. As of July 14, 2020, tx was ongoing in 3/38 pts (8%) in dose-escalation (all ongoing in cycle >24, 1 pt ongoing in cycle >40). In the dose-expansion, tx was ongoing in 15/35 pts (43%); of these pts, all were ongoing in cycle >21 and 1 was ongoing in cycle 30. The RP2D of avadomide was established as 3.0 mg formulated capsule. In the expansion part of the study, pts received a median of 15 tx cycles (range, 1-33), and median tx duration was 60 wk (range, 1-132). The most common (≥10%) grade 3/4 TEAEs were neutropenia, reported in 19 pts (54%) and thrombocytopenia, reported in 7 pts (20%). Sixteen pts (46%) had a serious TEAE; only pyrexia (11%) and sepsis (9%) occurred in >2 pts. The objective response rate (ORR) among the 35 pts with R/R FL in the dose-expansion part of the study was 71%, including 40% with a complete response (CR). The mDOR was 14.6 mo (95% CI, 14.6-not estimable [NE]), and mPFS was 16.4 mo (95% CI, 8.3-NE). The median duration of PFS follow-up in dose-expansion was 14.4 mo (range, 0.8-30.3). Two pts had CRs lasting 13.7 mo and 15.3 mo before discontinuation owing to receiving allogeneic stem cell transplantation. Response rates were similar among all 53 R/R FL pts in both parts of the study (dose escalation and dose expansion) and in high-risk (ER and/or DR) FL pts (Table).
Conclusions: Long-term follow-up results demonstrate that avadomide plus obinutuzumab has a manageable safety profile and durable responses in patients with R/R FL. The antitumor activity of cereblon modulators plus next-generation anti-CD20 antibodies in heavily pretreated R/R NHL warrants further investigation as a novel chemotherapy-free option.
Michot:Genentech: Research Funding; Abbvie: Research Funding; Forma: Research Funding; Eisai: Research Funding; Debiopharm: Research Funding; Daiichi Sankyo: Research Funding; Kyowa: Research Funding; AstraZeneca: Other, Research Funding; Gustave Roussy: Honoraria, Other: Abbvie, Aduro, Agios, Amgen, Argen-x, Astex, AstraZeneca, Aveo pharmaceuticals, Bayer, Beigene, Blueprint, BMS, Boeringer Ingelheim, Celgene, Chugai, Clovis, Daiichi Sankyo, Debiopharm, Eisai, Eos, Exelixis, Forma, Gamamabs, Genentech, Gortec, GSK, H3 bio, Research Funding; Janssen: Other, Research Funding; Celgene: Other; Mundi Pharma: Other; Eos: Research Funding; AZD: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Research Funding; Medimmune: Research Funding; Lytix Biopharma: Research Funding; Lysarc: Research Funding; Lilly: Research Funding; Astex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Argen-x: Research Funding; Amgen: Research Funding; Agios: Research Funding; Xencor: Research Funding; Sanofi: Research Funding; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Other; Exelixis: Research Funding. Doorduijn:Roche: Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company). Boccomini:SC Ematologia, ASOU Città della Salute e della Scienza di Torino, Turin, Italy: Current Employment. Kersten:Takeda: Research Funding; Miltenyi Biotech: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Janssen/Cilag: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); BMS: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); MSD: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Novartis: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Roche: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; Celgene: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; Kite/Gilead: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Amgen: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company). Chiappella:Servier: Honoraria; Takeda: Honoraria; Roche: Honoraria; Janssen: Honoraria; Gilead-Kite: Honoraria; Celgene: Honoraria; Iqone: Honoraria. Zinzani:Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSA Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics, Inc.: Honoraria, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kirin Kyowa: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Immune Design: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kyowa Kirin: Consultancy, Speakers Bureau; Eusapharma: Consultancy, Speakers Bureau; Portola: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Sandoz: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Immune Design: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Salles:Abbvie, Autolus, BMS/Celgene, Debiopharm, Genmab, Kite/Gilead, Epizyme, Janssen, Karyopharm, Morphosys, Novartis, F. Hoffmann-La Roche, Takeda: Consultancy; Abbvie, Amgen, Celgene, Gilead, Janssen, Kite, Morphosys, Novartis, Roche, Takeda: Other: Participation to educational events; Abbvie, Autolus, BMS/Celgene, Debiopharm, Genmab, Kite/Gilead, Epizyme, Janssen, Karyopharm, Morphosys, Novartis, Roche, Takeda: Membership on an entity's Board of Directors or advisory committees; Abbvie, Amgen, Celgene, Gilead, Janssen, Kite, Morphosys, Novartis, F. Hoffmann-La Roche, Takeda: Honoraria. Hentrup:Neuronetics, Inc: Current equity holder in publicly-traded company, Ended employment in the past 24 months; BMS Consultant: Current Employment. Rhee:I own stocks for publicly-traded companies: Current equity holder in publicly-traded company; Bristol-Myers Squibb: Current Employment. Hagner:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Klein:Roche: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. Pourdehnad:Celgene: Ended employment in the past 24 months, Patents & Royalties: Various CC-122 patents; Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties: Various CC-122 patents. Hege:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Patents & Royalties: numerous, Research Funding; Celgene (acquired by Bristol Myers Squibb): Ended employment in the past 24 months; Mersana Therapeutics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Arcus Biosciences (Former Board of Directors): Divested equity in a private or publicly-traded company in the past 24 months. Dobmeyer:BMS: Consultancy. Nikolova:Celgene, A Bristol-Myers Squibb Company: Current Employment, Current equity holder in publicly-traded company, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company). Ribrag:nanostring: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; gustave roussy comprehensive cancer center: Current Employment; servier: Consultancy; pharmamar: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; argenX: Research Funding; epizyme (EPZ): Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; EPZ: Honoraria, Membership on an entity's Board of Directors or advisory committees; AZD: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other; Infinity: Honoraria, Membership on an entity's Board of Directors or advisory committees.
This is a phase I study evaluating the safety and efficacy of avadomide in combination with obinutuzumab in patients with R/R B-cell NHL. Avadomide is an investigational agent and has not yet been approved in the US.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal